Nanotube water doesn't freeze - even at hundreds of degrees below zero
A new form of water has been discovered by physicists in Argonne's Intense Pulsed Neutron Source (IPNS) Division. Called nanotube water, these molecules contain two hydrogen atoms and one oxygen atom but do not turn into ice - even at temperatures near absolute zero.Instead, inside a single wall tube of carbon atoms less than 2 nanometers the water forms an icy, inner wall of water molecules with a chain of liquid-like water molecules flowing through the center. This occurs at 8 Kelvins, which is minus 509 Fahrenheit. As the temperature rises closer to room temperature, the nanotube water gradually becomes liquid.
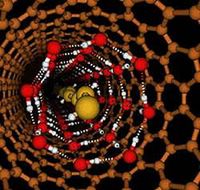
Image: New form of water in a nanotube. Water behaves differently when confined inside a long, narrow nanotube. The copper-colored exterior rings represent the carbon nanotube 1.4 nanometers across. The red and white interior cylinder is an icy wall with permanent hydrogen bonds shown in red; white represents oxygen. The interior chain is in constant motion. Yellow represents the hydrogen in the chain. Image by Christian J. Burnham, University of Houston.
Research partners at MER Corp., Tucson, Ariz., supplied the nanotube samples made of nearly pure carbon only one atom thick. Each tube was 1.4 nanometers across and 10,000 nanometers long; imagine a piece of dry, hollow spaghetti 200 meters long because the nanotube is 100,000 times longer than wide.
Nanotube water doesn't freeze - even at hundreds of degrees below zero



0 Comments:
Post a Comment
<< Home